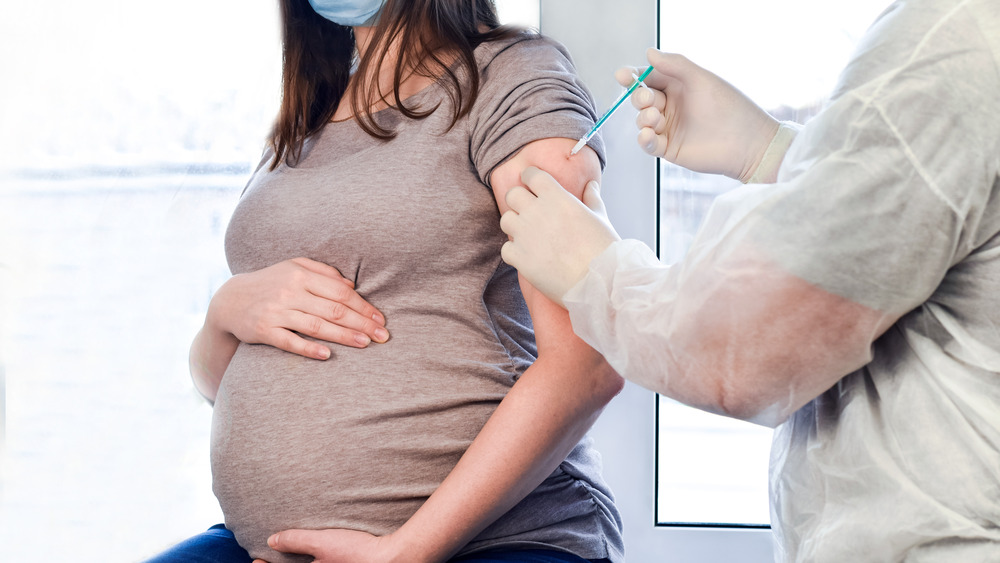
Why The First Baby Born In The US With COVID-19 Antibodies Is Significant
Three weeks after receiving her first dose of the Moderna COVID-19 vaccine, a frontline healthcare worker from Florida gave birth to a healthy baby girl (via CBS News). A sample of blood taken from the umbilical cord immediately after the birth revealed that the newborn had actually received COVID-19 antibodies from her mother. This newborn is believed to be the first documented case of mother-to-newborn COVID-19 antibody transmission post-vaccination.
The phenomenon of maternal immunity, or the transmission of maternal antibodies to a newborn, particularly those known as immunoglobulin G (IgG) antibodies, has been well established (via Nature Medicine). This passage of antibodies from a mother to her offspring is critical, as the newborn is unable to produce IgG antibodies until almost three and a half months after birth. In the meantime, it is the mother's immunity which protects the newborn from a whole host of viruses and bacteria.
While it has already been established that mothers who had contracted COVID-19 passed on antibodies to their newborns, this recent finding demonstrates a familiar finding — maternal transmission of antibodies from vaccinations (via Health). For instance, pregnant mothers vaccinated against the flu are known to pass on protective antibodies to their newborns.
Vaccine manufacturers to study safety and efficacy in pregnant women and children
News of the first newborn in the U.S. with maternal antibodies against COVID-19 is an encouraging finding, at a time when significantly more research is necessary to determine the safety and efficacy of COVID-19 vaccines in the pregnant population. Pregnant women were notably not a part of the late stage clinical trials for any of the vaccines authorized for emergency use in the U.S: the Pfizer, Moderna, or Johnson & Johnson vaccines.
Last month, however, Pfizer launched its own trial in the pregnant population, which it plans to end in early 2023. Johnson & Johnson has also followed with an announcement that it is planning on including pregnant women and their infants in future studies. Moderna, while tracking pregnant patients who have received the vaccine, is currently focusing on trials regarding safety and efficacy involving young children ages six months to 11 years old (via NPR).
While there is still a ways to go in regards to better understanding COVID-19 vaccines in the pregnant and under 16 year old demographics, one thing has become apparently clear — the available vaccines are quite promising in reducing severe COVID-19 infections and complications. To date, the United States has administered over 150,000,000 vaccines and is ramping up efforts to make all adults eligible for vaccination no later than May 1. In the meantime, continue to wear a mask in public, practice social distancing, and wash your hands regularly.