FDA Announces At-Home Flu And RSV Tests Are In The Works
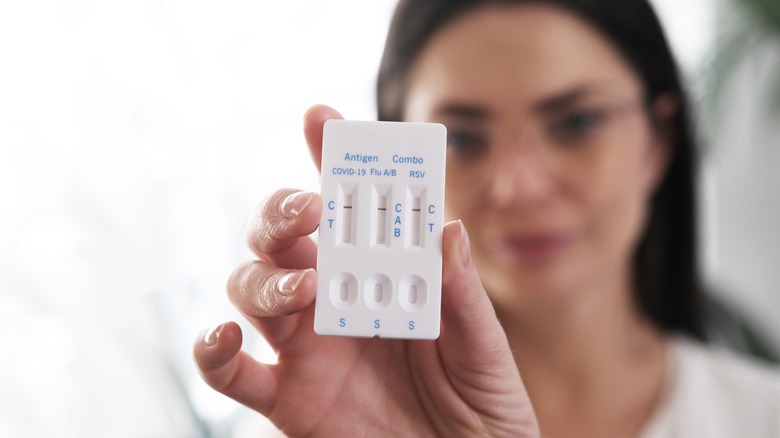
In mid-May of 2022, the U.S. Food and Drug Administration (FDA) released a public statement announcing their formal authorization of the first non-prescription COVID-19 nasal swab test that can not only detect COVID, but also pick up on the presence of RSV and the flu virus. The combination test allowed users to collect their sample at home before mailing it to LabCorp and receiving online test results.
While the test conveniently offered a partial at-home component, it still presented challenges to users, as reported via NBC News. Such challenges included cost and a waiting period for test results. However, Jeff Shuren, director of FDA's Center for Devices and Radiological Health, stated in the press release that the development of this combination test was a step in the right direction towards complete at-home tests. At the time, however, the agency had not confirmed whether they had any entirely at-home combination tests in the works (per NBC News).
Combination tests are in the development stages
Now, the FDA has announced that combination COVID, flu, and RSV at-home rapid tests are well on their way toward becoming available for the public, reports WebMD. Currently, the National Institutes of Health are working with developers in the creation of such combination respiratory illness tests. With pandemic protocols in place, seeking FDA authorization of combination tests is now simpler and less costly for developers. "We will be authorizing at-home flu and/or RSV tests that are multi-analyte with COVID," an FDA spokesperson confirmed with WebMD. "I can't tell you exactly when that would happen, but we are eager to do that."
The flu is estimated to have been responsible for at least 25 million cases of illness thus far this season, reports the U.S. Centers for Disease Control and Prevention (CDC). Although cases of both influenza and RSV have been on the decline since the start of 2023, experts state that the viruses continue to circulate (via NPR). In addition, the COVID-19 XBB.1.5 variant is now reported to make up about 66% of new cases, according to CDC data. In developing combination at-home rapid tests for all three respiratory illnesses, the FDA emphasizes their continued dedication to increasing health equity and accessibility for the public (via WebMD).