Read This Before You Get The COVID-19 Vaccine
The COVID-19 pandemic and subsequent vaccines have brought a whirlwind of information, some of it true, some of it not, and much of it developing in real time — at a time when most of us were completely unqualified to assess credibility or to parse what it actually meant in real life. As of this writing, three vaccines are available for people living in United States. While that's great news, it also means there's even more information — and it seems to be coming at us even faster.
There's also an increasing sense of urgency, with everyone from the President of the United States to your favorite celebrities imploring each and every one of us to go out and get vaccinated as soon as we're eligible. That can feel overwhelming until, of course, you get a handle on the facts. That's where we come in. Read this as you consider getting your COVID-19 vaccine.
Which COVID-19 vaccines have earned FDA approval?
At least six vaccines against COVID-19 have been developed or are in development as of April 2021 (via COVID-19 Real Time Learning Network). None are fully "FDA-approved," but three have been given "emergency use authorization" (EUA) by the U.S. Food and Drug Administration (FDA), WUSA9 explained.
The granting of an EUA means that members of the public may receive the vaccine in advance of FDA approval. FDA approval of a drug means that "data on the drug's effects have been reviewed by CDER [the FDA's Center for Drug Evaluation and Research], and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population," according to the FDA.
Likewise, for an EUA to be issued for a vaccine, the "FDA must determine that the known and potential benefits outweigh the known and potential risks of the vaccine," the FDA explains in its EUA information sheet. As of this writing, the three COVID-19 vaccines that are subject to an EUA are the ones by Pfizer-BioNTech (aka Pfizer), Moderna, and Janssen Pharmaceuticals Companies of Johnson & Johnson (aka Johnson & Johnson), per the Centers for Disease Control and Prevention.
Was the COVID-19 vaccine rushed through FDA emergency use authorization?
"In a first for vaccine development, two COVID-19 vaccines were created, evaluated and authorized for emergency use in under a year," the University of Nebraska Medical Center (UNMC) pointed out in a February 2021 article addressing the very issue of whether the COVID-19 vaccines were "rushed." None of that is to say that corners were cut. "Despite the fast timeline, these vaccines went through the appropriate clinical trials, just like other vaccines before," UNMC went on to say. Further, it's not as if the FDA just rubber-stamped the vaccines and went on to doing other things. In fact, the granting of an EUA is conditioned on continuing study and reporting on the vaccines (via FDA).
Additionally, one of the vaccines for which the FDA has not issued an EUA is the one developed by AstraZeneca in conjunction with Oxford University in the U.K. The reason? Large-scale phase 3 clinical studies are still in progress. Thus, the FDA cannot say that the AstraZeneca vaccine is both safe and effective. And it's worth noting that the FDA is waiting for safety and effectiveness confirmation while other nations have gone ahead to authorize its use (via Vox).
Aren't vaccines supposed to take a lot longer to develop?
Historically, it's taken as long as 10 to 15 years of extremely costly research in order to develop and authorize a vaccine for use in the U.S., according to History of Vaccines. So how is it even possible that three COVID-19 vaccines could have been developed and proven effective and safe enough for emergency use authorization within such a short time frame?
First, "pauses between steps, often needed to secure funding, have been shortened, or eliminated, and in some cases, steps are being carried out in parallel to accelerate the process, wherever that is safe to do," according to the World Health Organization. And that's been made possible by the commitment of vast resources by governments and businesses, according to Healthline.
Second, a lot of the legwork that might have been required to develop a vaccine had already been accomplished during earlier research of previous vaccines (via Healthline). And by the time the novel coronavirus reared its spiky head, much was already known about other coronaviruses, which were first described in a medical journal in 1965, as highlighted by a 2020 paper in The BMJ.
What measures are being taken to ensure the COVID-19 vaccines are safe?
"Like all vaccines, COVID-19 vaccines are going through a rigorous, multi-stage testing process," which includes large-scale randomized, controlled clinical trials designed to identify adverse affects among tens of thousands of volunteers," according to the World Health Organization (WHO). But the evaluation of the vaccines' safety does not stop there.
"Once a clinical trial shows that a COVID-19 vaccine is safe and effective, a series of independent reviews of the efficacy and safety evidence is required," the WHO explained. And before a country such as the U.S. even gets to consider whether to authorize use of a vaccine, a panel of experts assembled by the Organization has to review all the existing data and decide whether to recommend the vaccine's use.
By the time a vaccine has been introduced into the public, multiple systems are in place for monitoring its safety, the CDC confirmed. These include public databases of "adverse events" that health care providers and others can access and contribute to. You, yourself, can support the effort by reporting adverse events to the Vaccine Adverse Event Reporting System (VAERS), the vaccine safety monitoring system managed by CDC and FDA, among others.
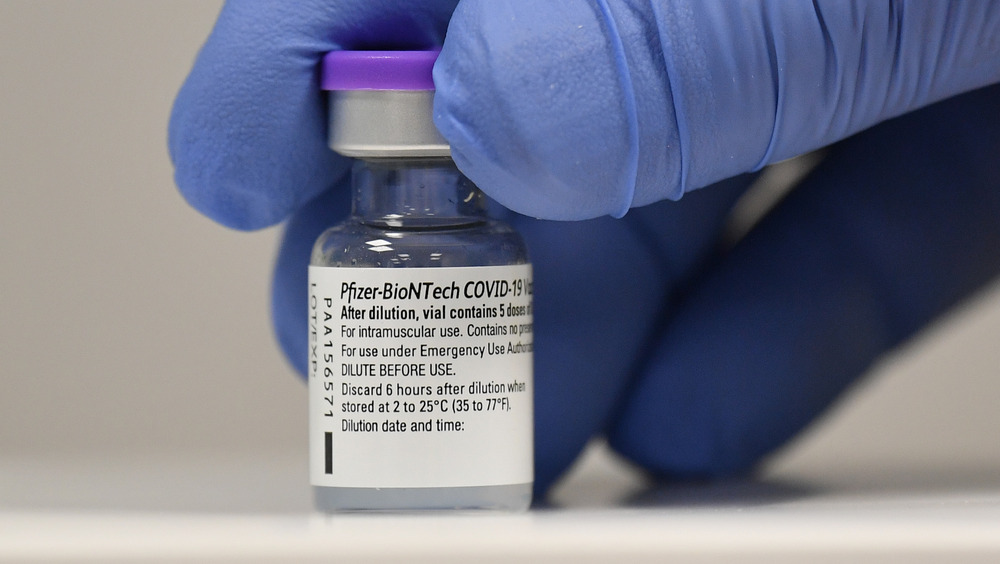
What should you know about the Pfizer vaccine?
The vaccine we know as the Pfizer vaccine was developed by Pfizer, Inc. in conjunction with BioNTech (via CDC). As of this writing, the Pfizer vaccine regimen consists of two injections to the muscle in the upper arm, spaced 21 days apart.
There are a number of different types of vaccines, categorized by how they work to protect the human body from infection, but the Pfizer vaccine is what's known as an "mRNA" vaccine. MRNA vaccines are a new type of vaccine, according to the CDC. They work by "[teaching] our cells how to make a protein — or even just a piece of a protein — that triggers an immune response inside our bodies. That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies."
The Pfizer vaccine is recommended for people aged 16 and older. It does not contain eggs, preservatives, or latex, which could be important to people with allergies. Side effects of the Pfizer vaccine include pain, redness and swelling at the injection site and overall flu-like symptoms.
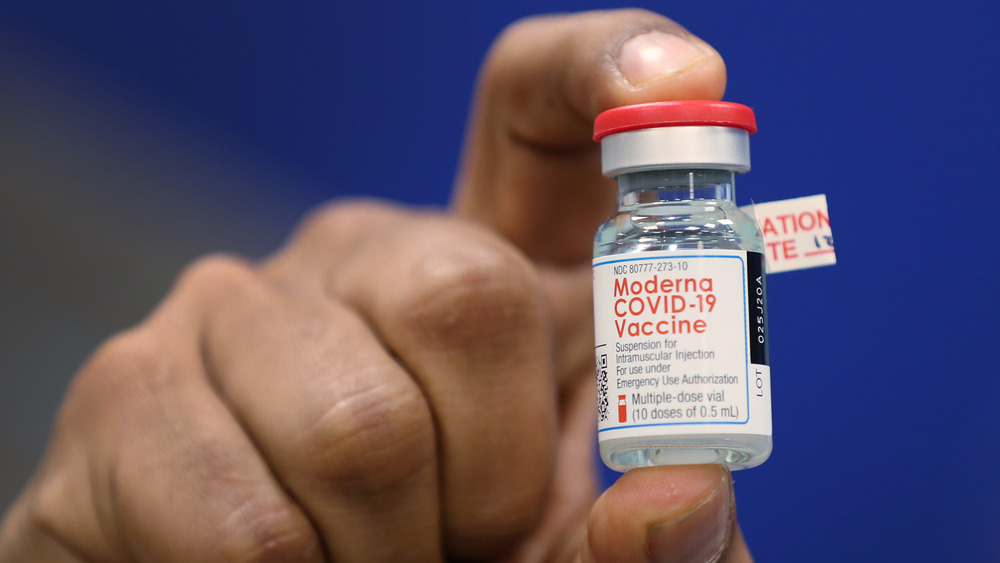
What should you know about the Moderna vaccine?
The vaccine known as the Moderna COVID-19 vaccine was developed by ModernaTX, Inc., according to the CDC. It currently consists of two injections spaced 28 days apart. Like the Pfizer vaccine, the Moderna vaccine falls into the category of mRNA vaccines, which "give instructions for our cells to make a harmless piece of what is called the 'spike protein.' The spike protein is found on the surface of the virus that causes COVID-19," according to the CDC's information sheet on mRNA vaccines.
The Moderna vaccine is indicated for people aged 18 and older. It contains no eggs, preservatives, or latex, per the CDC. Common side effects of the Moderna vaccine include pain, redness and swelling at the injection site, and flu-like symptoms.
One potentially significant difference between the Moderna vaccine and the other authorized vaccines is that it's seemingly more likely to cause discomfort at the injection site. This phenomenon has a name: Moderna arm (or sometimes COVID arm), according to USA Today. Like the other side effects, though, it resolves quickly in most cases.
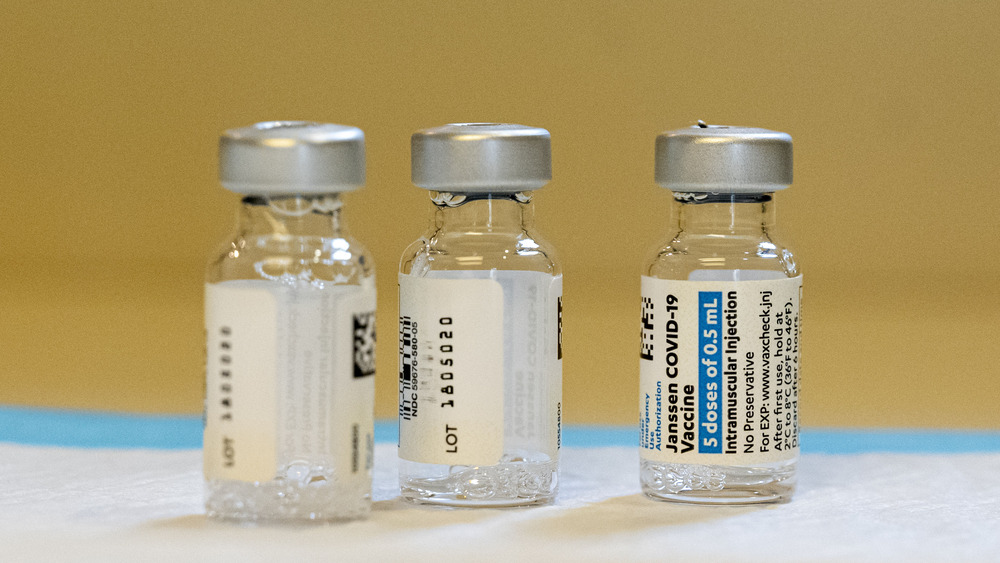
What should you know about the Johnson & Johnson vaccine?
The vaccine we know as the Johnson & Johnson COVID-19 vaccine (or the Janssen COVID-19 vaccine) was developed by the Janssen Pharmaceuticals Companies of Johnson & Johnson (via CDC). As of this writing, this vaccine regimen consists of one injection to the upper arm. The Johnson & Johnson vaccine is not an mRNA vaccine. Rather, it falls into the category known as viral vector vaccines, per the CDC.
"Viral vector vaccines use a modified version of a different virus (the vector) to deliver important instructions to our cells," the CDC's information sheet on viral vector vaccines. "For COVID-19 viral vector vaccines, the vector (not the virus that causes COVID-19, but a different, harmless virus) will enter a cell in our body and then use the cell's machinery to produce a harmless piece of the virus that causes COVID-19." This then causes the immune system to produce antibodies and fight what it thinks is an infection.
This one-dose vaccine is recommended for people aged 18 and older. Like the mRNA COVID-19 vaccines, it contains no eggs, preservatives, or latex. Typical side effects of the Johnson & Johnson vaccine include pain, redness and swelling at the injection site, as well as flu-like symptoms.
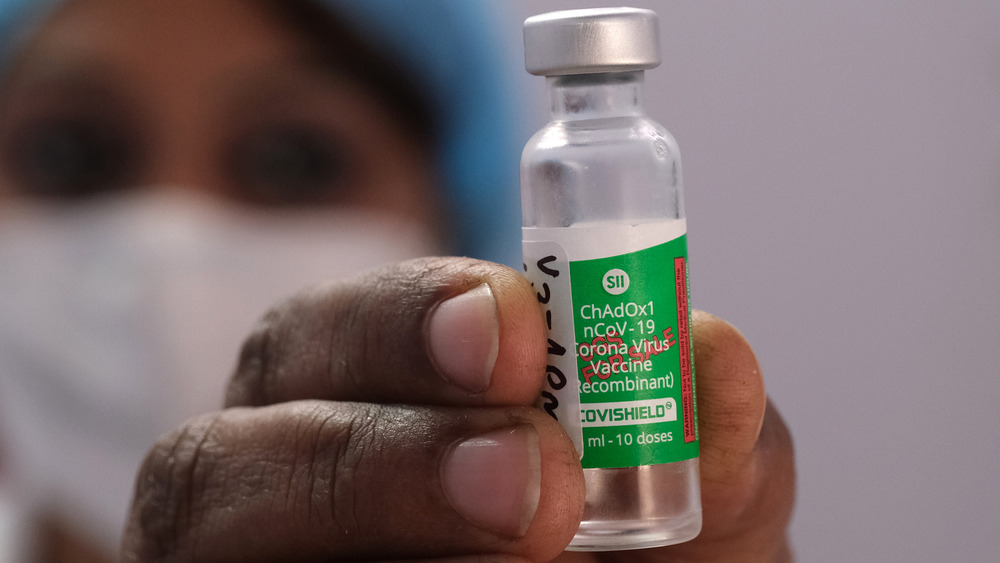
What about the AstraZeneca vaccine?
The vaccine known as the AstraZeneca COVID-19 vaccine was developed by pharmaceutical company AstraZeneca in conjunction with the U.K.'s Oxford University, according to the World Health Organization (WHO), which notes that "the recommended dosage is two doses given intramuscularly (0.5ml each) with an interval of 8 to 12 weeks." As of this writing, two versions of the AstraZeneca vaccine have been "listed for emergency use by WHO," but not in the U.S., according to the CDC.
For those who have doubts about the FDA's emergency authorization process with regard to the vaccines that have been granted emergency authorization, it is worth noting here that the FDA does consider the fact that the AstraZeneca vaccine has been approved for use in other countries as persuasive, according to Forbes. Nevertheless, the FDA has opted to stand down while concerns over the AstraZeneca vaccine's safety, including whether it can cause a blood clot that leads to bleeding on the brain, are hashed out among scientists (via The Washington Post).
How effective are the FDA-authorized vaccines at preventing COVID-19?
The Pfizer, Moderna, and Johnson & Johnson COVID-19 vaccines are all "effective at preventing COVID-19," according to the CDC. "COVID-19 vaccines help protect people who get vaccinated from getting sick or severely ill with COVID-19 and may also help protect people around them." As of this writing, the COVID-19 vaccines authorized for emergency use in the U.S. are considered by the CDC to be an "important tool" to halt the COVID-19 pandemic, but their efficacy continues to be monitored and evaluated.
Specifically, the Pfizer vaccine — after both doses were given — was proven to be 95 percent effective at preventing COVID-19 infection in clinical trials, according to the CDC's Pfizer vaccine fact sheet. In clinical trials, the Moderna vaccine was proven 94.1 percent effective at preventing COVID-19 infection, and the data suggests that where COVID-19 has been diagnosed, fewer people have been admitted to the hospital (via CDC).
The single-dose Johnson & Johnson vaccine was proven 66.3 percent effective at preventing COVID-19 infection in clinical trials, and may, based on preliminary evidence, protect against asymptomatic infection, according to the CDC's Johnson & Johnson/Janssen vaccine fact sheet.
If you are vaccinated against COVID-19, can you still catch or spread the coronavirus?
There is an important distinction between the novel coronavirus, SARS-CoV-2, and the illness it causes, COVID-19, Dr. Katherine O'Brien explained to the World Health Organization in January 2021. Dr. O'Brien revealed, "The clinical trials demonstrated that these vaccines protect people against disease. What we don't know yet from the clinical trials is whether or not the vaccines also protect people from just getting infected with the SARS-CoV-2 virus and whether or not it protects against transmitting to somebody else."
In other words, it is possible that the COVID-19 vaccines protect you from the illness, COVID-19, but not from the virus itself. And if that is the case, it might still be possible to transmit the virus to others. As of April 2021, we simply don't quite know. As such, experts are taking the position that you should operate from the assumption that after you are vaccinated, you may still be able to transmit the coronavirus to others, including people at high risk.
How long will the immunity from your COVID-19 vaccine last?
As of April 6, 2021, a total of 108,301,234 people in the U.S. have had at least one dose of a COVID-19 vaccine, and over 63 million people have been fully vaccinated, according to the CDC. Among the things the CDC is still getting its arms around, though, is "how long COVID-19 vaccines protect people."
That said, Pfizer reported on April 1, 2021 that a follow-up analysis demonstrates the Pfizer vaccine offers 91.3 percent "vaccine efficacy" for up to six months after the second dose. In addition, according to Albert Bourla, Pfizer's chairman and chief executive officer, that applies to the newer South Africa variant, thus offering "further confidence in our vaccine's overall effectiveness."
Immunity conferred by the COVID-19 vaccines may last far longer than six months, but that is the "longest volunteers in the trials have been followed to see what their immunity is," Scott Hensley, an immunologist and vaccine expert at the University of Pennsylvania, told CNN in early April 2021. With regard to the Pfizer and Moderna vaccines, clinical studies addressing the need for boosters are ongoing as of this writing.
Apart from the routine side effects, what are the risks associated with the COVID-19 vaccines?
It is normal to experience injection site soreness and general malaise as side effects after receiving the COVID-19 vaccine, and these are generally understood to signify the vaccine is doing its job of sensitizing your immune system to the identified threat (via CDC). However, there is a "remote chance" of a severe allergic reaction, which according to Pfizer, Johnson & Johnson, and Moderna's respective FDA vaccine recipient fact sheets "would usually occur" within a few minutes to one hour after receiving a dose. Signs of a severe allergic reaction include difficulty breathing, facial swelling, rapid heartbeat, full-body rash, dizziness, and weakness.
Due to this remote chance, it's recommend that you wait for some period of time after receiving your vaccination before leaving the premises. If you suspect you are having an allergic reaction, you should immediately contact 9-1-1 or go to the nearest hospital.
Can you take medication before your COVID-19 vaccine?
Most people will not need to "avoid, discontinue, or delay medications for underlying medical conditions" around the time of their COVID-19 vaccination (via CDC). However, if you're taking an immune-suppressing medication, it's advisable to speak to your healthcare provider to determine best practices.
Some people may wish to take an over-the-counter painkiller to prevent some of the vaccine's potential side effects. However, "it is not known how these medications might affect how well the vaccine works," the CDC revealed. Therefore, it is best to refrain from taking such medications just before your vaccine. If you happen to be taking any of these medications regularly for other reasons and as directed by a doctor, you may keep taking them before getting vaccinated. If after receiving your COVID-19 vaccine your side effects are causing you discomfort, please speak to your doctor about what you can take for relief, the CDC advised.
Note: "It is also not recommended to take antihistamines before getting a COVID-19 vaccine," whether to prevent an allergic reaction or otherwise, per the CDC. Nor should you get the COVID-19 vaccine at the same time as any other vaccine.
How do you get the COVID-19 vaccine?
President Joe Biden's goal is to open COVID-19 vaccination appointments for all American adults by April 19, 2021, according to The Washington Post. As of this writing, whether you are eligible for the vaccine depends upon your state of residence.
To determine if you can get vaccinated in your area, you can contact your state health department. It is likely you'll be asked to take an eligibility quiz before signing up for the vaccine. For example, if you are scheduling an appointment with VAMS (the Vaccine Administration Management System), a determination about your eligibility will be made before you can proceed to scheduling an appointment (via VAMS).
Assuming you are eligible to get a COVID-19 vaccine, you can find the one nearest to you by visiting VaccineFinder.org. In addition, you can check the websites of your local pharmacies to see if they are scheduling appointments. Your state health department will also be a good source for this information. Many vaccine providers use online scheduling systems, and many explain what you need to do if the vaccine they are offering requires a second appointment.
What should you expect at your COVID-19 vaccine appointment?
After you schedule your COVID-19 vaccination appointment, you should pull together any documentation that you have been told by the vaccine provider to bring with you, according to the CDC.
If you do not have photo ID that shows that your reside in the state where your vaccination is to take place, you should discuss that with the vaccination center. When you arrive at your COVID-19 vaccination appointment, you should be wearing a mask and also be prepared to practice social distancing. Some providers provide you with a vaccination record card in advance. You'll need to bring that to your appointment. If not, you should be given one at your appointment, which you should keep safe.
So, how much will this all cost you? "The federal government is providing the vaccine free of charge to all people living in the United States, regardless of their immigration or health insurance status," the CDC confirmed. You may, however, be asked for your insurance information. This is permissible and does not mean you will be charged for the vaccine, any related time spent at the vaccine center, or otherwise. However, your insurance company may be willing to reimburse the provider, per the CDC.
Do you have any choice about which COVID-19 vaccine you get?
As Five Thirty Eight put it, "People at the tail end of the vaccination priority list will likely be able to browse a veritable vaccine buffet." However, if you are eligible as of this writing, you'll probably end up getting whatever COVID-19 vaccine is available at your vaccination center on the day you get vaccinated. Five Thirty Eight pointed out that this is not bad news because all of the vaccines that have been authorized by the FDA for emergency use have been deemed effective and safe.
Plus, the CDC advises that "you should get any COVID-19 vaccine that is available when you are eligible. Do not wait for a specific brand. All currently authorized and recommended COVID-19 vaccines are safe and effective, and CDC does not recommend one vaccine over another."
If your vaccine requires a second dose, there is a minute possibility that you could receive a different COVID-19 vaccine at your final appointment, according to the Cleveland Clinic. However, the CDC has stated that switching between COVID-19 vaccine regimens should be done only in "truly exceptional situations" such as if the first dose vaccine is no longer available (via Cleveland Clinic).
Are there any underlying conditions that would preclude you from getting the COVID-19 vaccine?
Certain underlying conditions make the prospect of becoming infected with the novel coronavirus far more dangerous. Thankfully, none of those conditions preclude you from getting the COVID-19 vaccine. In fact, the CDC has pointed out that having any of these conditions makes it even more important that you get the COVID-19 vaccine as soon as you can.
What could preclude you from getting the vaccine is having an allergy to any of the vaccine's ingredients. However, having allergies in general should not keep you from getting the vaccine, according to the Cleveland Clinic.
If you are pregnant, you can still consider getting the COVID-19 vaccine as experts believe it does not pose any specific risk for pregnant women. However, since knowledge about COVID-19 is developing in real time, a conversation with your health care provider may prove beneficial. If you believe you may be in any group of people for whom the COVID-19 vaccine may be inadvisable, please visit this CDC resource for more information.
If you've had COVID-19, should you get the COVID-19 vaccine?
The notion of "herd immunity" refers to a population being indirectly protected from an infectious disease either as a result of vaccination or as a result of developing immunity through previous infection, according to the World Health Organization. Since the notion of herd immunity includes immunity through past infections, some might assume that if they tested positive for COVID-19, they must be immune. That is not the case, however.
The CDC recommends vaccination regardless of whether you have already had COVID-19 because it is not known how long you will be "protected from getting sick again after recovering." However, if you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine. If you aren't certain which treatments you received, your doctor will likely be able to give you that information.
Should you expect your life to change after getting the COVID-19 vaccine?
Even after you get your COVID-19 vaccine, your life may not change all that much, at least not at first, according to University of Chicago Medicine (UCM). There are several reasons, and they are interconnected. Although the COVID-19 vaccine reduces your risk of contracting COVID-19, it does not reduce that risk to zero. In addition, even after immunization, you may be capable of catching the virus and spreading it to others.
Finally, the COVID-19 vaccines are most effective when everyone has gotten one, or at least when enough people have gotten it that we have achieved "herd immunity" (also known as "population immunity"). That said, it is not currently known what percentage of the population will need to be immunized before we reach herd immunity (via CDC). In fact, it appears that we won't know we have reached it until we reach it (via UCM).
However, "America's doctor," Dr. Anthony Fauci, estimated "somewhere between 70, 75, maybe 80%" when speaking with CNBC in late December 2020. As of this writing, though, the CDC recommends continuing on with masking and social distancing, but with several very specific exceptions, which are laid out in detail here.
How can you know what the long-term effects of the COVID-19 vaccine will be?
Given how relatively little time we've spent dealing with COVID-19, we likewise have spent even less time with the vaccine meant to protect against it — and with that lack of time also comes a general lack of knowledge. Simply put, it's altogether impossible to say for sure what long-term effects, if any, the vaccine will have, because not enough time has passed to even begin to answer that question.
However, doctors and scientists are still studying this subject, and it is partly behind the FDA's requirement that vaccine makers continue to follow up on their research by collecting data on adverse effects. Moreover, the FDA "undertakes a rigorous evaluation of the scientific information through all phases of clinical trials, which continues after a vaccine has been approved by FDA or authorized for emergency use."
The good news is that even with our comparatively small amount of data, the science seems to indicate that we don't have much to worry about in this case. According to the Children's Hospital of Philadelphia (CHOP), although the "rules of science" forbid scientists from saying that long-term effects can't happen, "the evidence is strong that mRNA vaccines will not cause long-term harm." In other words, the Pfizer and Moderna vaccines do not pose any known long-term risks. While CHOP made no reference to the Johnson & Johnson vaccine (the report was published prior to the vaccine's emergency authorization), the CDC has pointed out that viral vector vaccines, in general, have been well-studied and deemed safe for use in the past, including in response to Ebola.