The Real Reason There Are Concerns Over Merck's New COVID-19 Pill
On November 30, FDA advisors will review clinical data and make a decision about whether or not to recommend emergency use authorization (EUA) for a new early-treatment antiviral pill for COVID-19. The drug in question is called molnupiravir, and it has been discovered in recent years to be an effective antiviral against several different types of viruses (via CNN). So naturally, scientists are very interested in seeing how effective it is against the virus-de-jour, COVID-19.
The most recent study data released by Merck, which co-owns molnupiravir along with Ridgeback Biotherapeutics, showed that when given as an early treatment to patients with mild-to-moderate COVID-19 symptoms, the drug reduced the rate of hospitalization or death by about 30 percent — down from the 50 percent originally observed (via Yahoo). Despite this drop in estimated effectiveness, many researchers are still optimistic that the drug will receive FDA authorization. According to Dr. David Boulware, an infectious disease researcher at the University of Minnesota, "It's still a 30 percent effect, which is still good for a high-risk population" (via The Washington Post).
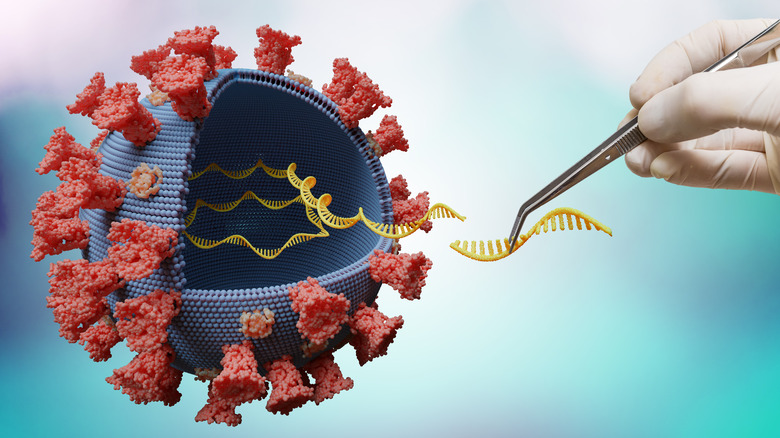
Molnupiravir works by interfering with the virus' RNA
There are some safety concerns about molnupiravir. The drug works by mimicking RNA by introducing errors into the virus's genome, which interferes with how the virus then replicates itself (via CNN). That's great if it sticks strictly to attacking the viral RNA. But there are concerns that the drug could interfere with the genome of other cells in the body instead — a result described as an "off-target effect."
Elizabeth Campbell, a Rockefeller microbial biochemist who co-wrote an article about molnupiravir in the science journal Nature, wrote, "The potential off-target effects will require further investigation" (via CNN). Another concern is that the drug could potentially harm the developing fetus in pregnant women. Campbell told CNN, "If I were pregnant, I wouldn't take it. I'm guessing that the FDA will not let molnupiravir be used in pregnant women." A third concern is that the pills could lead to further coronavirus mutations that turn out to be vaccine-resistant.
All in all, though, researchers are optimistic that molnupiravir could prove to be a helpful tool in physicians' limited toolkits to fight COVID-19 infections. Dr. Eric Rubin, an infectious disease expert at the Harvard T.H. Chan School of Public Health, told CNN, "We should worry about the dangers to pregnant women and the dangers of developing resistance. But if we have a drug that works, we want that drug. We just have to figure out how best to use it given its limitations."