Crohn's Disease Explained: Causes, Symptoms, And Treatments
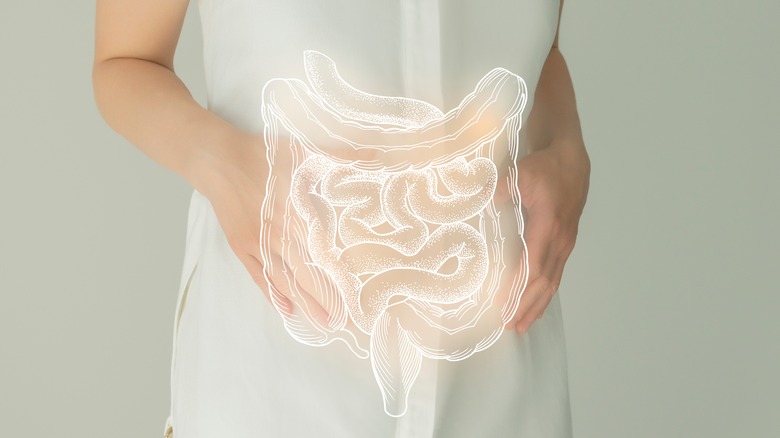
Crohn's disease (CD), along with ulcerative colitis (UC), is an inflammatory bowel disease (IBD) that causes chronic irritation and pain in the intestines (via The Crohn's and Colitis Foundation). The disease can at times be silent (no symptoms) during remissions to debilitating during flare-ups, and is characterized by digestive symptoms such as stomach pain and diarrhea as well as symptoms outside of the digestive system such as fatigue, joint pain, and rashes on the skin. Unlike UC, Crohn's can impact certain parts of the digestive tract (which in its entirety goes from the mouth to the anus) and not others, which is why it is broken down into five types, distinguishing where the inflammation and damage was discovered and the treatments recommended. Ileocolitis is the most common type and affects the end of the small intestine as well as the large intestine.
In addition to the daily trials a sufferer may experience, they may also be at an increased risk for other health conditions like colorectal cancer (via WebMD). Research shows varied analysis of risk, but experts agree that managing CD, along with getting regular screenings like colonoscopies, minimizes this risk.
Unfortunately there is no cure for CD, but improved monitoring and therapies including medication, diet, and surgery continue to emerge as researchers look deeper into the intricacies of the disease, making longer remission times a reality.
Causes of Crohn's Disease
Despite the research conducted, the precise cause of CD still remains unclear, and there appear to be multiple factors that contribute to increased risk and development of the disease. According to a 2016 research review, there are three phases of the development of the disease: the catalyst (typically a gastrointestinal infection), an impaired immune response to the infection, and a chronic inflammatory immune response (via F1000 Research). The instigating infection is typically caused by a virus such as E. coli, Norovirus, or Campylobacter, and has a more detrimental effect on some people than others, potentially due to mutations in certain genes that cause an increased susceptibility to CD.
Typically when the body is faced with a pathogen, such as a virus, it sets in motion an immune response to fight the infection (via WebMD). Once the pathogen is gone, the body can typically end that response. Unfortunately, in some individuals this process goes haywire and the immune system may fight things like beneficial gut bacteria, and/or the inflammatory response that was necessary to fight the initial infection doesn't stop once the pathogen is destroyed. That persistent inflammatory response has the potential to cause damage to the intestinal tract, leading to CD.
Environmental factors such as smoking also contribute to the development of the disease. Smoking reduces the intestinal blood flow that is necessary for the acute inflammatory response — an important part of the body's fight against infection (via F1000 Research).
Most common symptoms
According to WebMD, symptoms of CD depend on the part of the digestive system that is inflamed. The problem area(s) can be determined by working with a gastroenterologist — they will run a series of tests such as a colonoscopy, MRI, and blood work to diagnose CD and its location in the GI tract. Most commonly, those with CD experience symptoms of their lower GI tract like cramping, frequent diarrhea, and blood in their stool, as well as a decreased appetite, unintended weight loss, and fatigue. If you're experiencing any combination of these symptoms regularly, it's important to connect with your doctor asap because CD can cause additional concerns such as abscesses from bacterial infections, fissures in the anal lining, fistulas or ulcers in the intestine, and strictures that can partially block passages in the GI tract.
While the GI symptoms of CD can be devastating on their own, this disease can also cause complications outside of the digestive system, referred to as extraintestinal manifestations or EIMs (via Gastroenterology & Hepatology). EIMs can be seen in the joints, bones, eyes, and skin, as well as organs like the liver, lungs, and pancreas. How can inflammation in the digestive system affect other areas of the body? The Journal of Crohn's and Colitis explains that EIMs can be a continuation of the inflammatory response in the GI system, or may be independent but caused by the same genetic or environmental risk factors as CD.
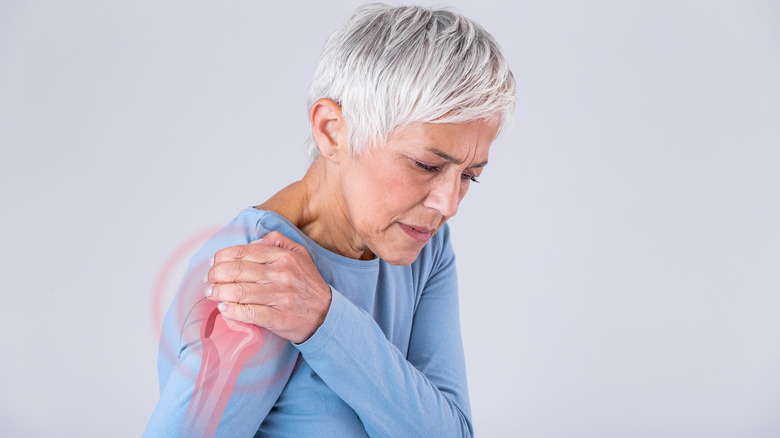
Joint pain and inflammation
Joint pain is the most common EIM and is found in up to one third of IBD patients, occurring more often with Crohn's than ulcerative colitis (via The Crohn's & Colitis Foundation). While some EIMs require treatment separate from CD, pain in smaller joints such as the wrists and ankles can often be addressed by treating the inflammation in the digestive system, according to Dr. David Rubin, the co-director of the Digestive Diseases Center at The University of Chicago Medicine (via Gastroenterology & Hepatology). Pain and inflammation in larger joints such as shoulders, knees, and the spine typically require additional treatment. Patients should work with their medical team to determine the best course of action, as common pain relief drugs such as NSAIDs (aspirin and ibuprofen) may exacerbate GI tract inflammation and irritation, according to The Crohn's & Colitis Foundation. Additionally, Dr. Rubin goes on to explain that a detailed patient history is important in determining the cause of the joint pain, because in addition to its direct correlation to CD, it can also be caused by some medications used to treat IBD, or by withdrawal from medications such as steroids.
Risk of depression and anxiety
There appears to be a higher prevalence of depression and anxiety in IBD patients than in the general public. One study reported around 40% of participants with IBD had depression, about 30% had anxiety (via Inflammatory Bowel Diseases). Depression is characterized by persistent sadness, hopelessness, difficulty concentrating, fatigue, and loss of interest in once enjoyable activities, while anxiety involves feelings of nervousness, restlessness, worry, and irritability. Both conditions can alter quality of life and outcomes of disease management.
While there was once a school of thought that suggested mental illness and stress could cause or contribute to chronic digestive diseases, there is no current scientific evidence that this is the case (via The Crohn's & Colitis Foundation) — mental conditions don't lead to IBD. However, the reverse may be the case: It is likely that the impact to quality of life, financial burdens, increased stress and worry about the future, chronic pain, and the overwhelm that comes with managing a chronic disease can lead to depression and anxiety. It's also possible that mental health can impact a person's ability to manage the disease, and that stress may contribute to flare-ups.
Risk of malnutrition
Malnutrition is a condition that develops because a person has not received the right amount or balance of calories or nutrients, and as a result is underweight, overweight, or has developed other health problems as a result of those deficiencies (via the World Health Organization). Crohn's patients are at a higher risk of malnutrition in the form of low body weight, nutrient deficiencies, and reduced muscle mass due to frequent diarrhea (loss of electrolytes), food avoidance to prevent symptoms, reduced appetite, malabsorption of nutrients, and some medications (via The Crohn's & Colitis Foundation). Malnutrition can impact the ability to manage CD and cause more complications post-surgery, longer hospital stays, and poorer quality of life.
A 2021 study reported that no one malnutrition assessment tool is ideal for IBD despite the high need for such an assessment, especially pre-surgery. The researchers developed an assessment tool called NS-IBD that takes into account factors such as current symptoms like vomiting, diarrhea, pain, and appetite, in addition to surgery history, body weight, BMI, and unintended weight loss. While the assessment requires more research to substantiate, it offers a simple, highly sensitive way to determine if a patient is malnourished and at a greater risk for poor outcomes.
Goals of treatment
Just as symptoms and severity of Crohn's disease vary from person to person, so do treatment plans. Current research suggests that management of CD should be individualized and involve treatment not just of symptoms, but of the inflammation and damage to the digestive tract (via Mayo Clinic). A 2019 study recommends a "treat-to-target" approach, which focuses on an individualized goal or target, consistent observation, and a change in treatment plan if needed to reach the goal. The study argues that in the past patients have received inadequate treatment when symptom remission was the main goal rather than healing the GI tract.
It's important to have a medical team that will work with the patient to determine a treatment plan based on disease severity, location of disease, responses and side effects to medications, and will provide ongoing clinical assessments — every 3 months during flare-ups and every 6-12 months during remission. According to researchers, it is also important to assess a patient's quality of life alongside treatments and evaluations in order to embrace a more holistic protocol that takes into account emotional and social health in addition to physical wellness (via Quality of Life Research).
Common medications
The National Institute of Diabetes and Digestive and Kidney Diseases offers a breakdown of common medications used to treat the symptoms of Crohn's disease. Aminosalicylates, steroids, and immunomodulators help to reduce immune activity and inflammation, which can temporarily suppress symptoms and help patients go into remission. But like any medication, there are side effects and some questions about long-term use.
According to the Journal of Gastroenterology, side effects of IBD medications, such as headaches, abdominal pain, diarrhea, and nausea, are common and have the potential to impact disease management and decrease quality of life (which is already affected by the disease itself). Experts agree that patients should be closely monitored to determine if there's a need for a change in dosing or medication, and some treatments should be used for only short periods of time (via Mayo Clinic). The newest medications being used for IBD, anti-TNF agents, are aimed at activating the healing of the lining of the digestive tract rather than merely treating symptoms. Anti-TNF drugs help to stop the body from making too much tumor necrosis factor (TNF), which leads to high levels of inflammation in the body, and certain anti-TNF drugs can help heal wounds in the digestive tract (via Journal Crohn's and Colitis). The combination of reduced inflammation and wound repair offers a greater chance of healing the GI tract.
When is surgery necessary?
According to The Crohn's & Colitis Foundation, because CD is a lifelong, often progressive disease, surgery may become necessary for patients due to complications such as a stricture that is blocking the intestine, or a perforation of the bowel (hole in the wall of the intestine). Some patients also may choose to have surgery to improve quality of life when other therapies like medications aren't working, or the side effects are substantial.
According to research published in Gastroenterology & Hepatology, surgery is a difficult decision for some patients and their medical teams because while early intervention is helpful for some, excessive treatment can be detrimental due to the possibility of complications such as infections, scarring, and the need for additional surgeries. Like each treatment presented to a CD patient, surgery should be assessed based on the seriousness of the disease, damage to the intestines, and the presence of a complication such as intestinal bleeding or a stricture. Experts believe that the primary goal should be preservation of the bowel, and laparoscopic surgery is preferable to open surgery when possible in order to limit post-surgery complications and pain. Advances in other treatments such as anti-TNF medications are making it possible for many CD patients to avoid surgery.
How does diet impact Crohn's disease?
There's no question that CD will affect a person's diet simply due to the disease's impact on the digestive system, but there is no one diet that has been shown to prevent, cure, or improve CD. While some patients report success with diets such as the Specific Carbohydrate Diet, Low FODMAP Diet, or anti-inflammatory diet, organizations like The Crohn's & Colitis Foundation do not support a specific diet. Instead they recommend tracking intake and symptoms and avoiding any foods that seem to exacerbate symptoms, as well as working with a nutrition professional to create a diet that works best for you based on factors such as trigger foods, symptoms, remission versus flare-ups, and nutrient deficiencies.
Unfortunately, it doesn't appear that most CD patients feel they have adequate access to nutrition information or professionals. A 2016 study surveyed medical professionals and patients to assess their knowledge of nutrition in relation to IBD (via Inflammatory Bowel Diseases). Only 27% of patients reported having good knowledge of nutrition and felt that resources and access to nutrition professionals were lacking. Nutrition education may be an area of CD treatment protocols that could have the potential to improve outlook for patients and improve disease outcomes, especially as treatment goals continue to move toward healing the gut rather than merely treating symptoms.
Review of IBD diets
The type of diet that will most benefit a patient with CD is dependent on the individual and the current status of their disease. A patient experiencing significant weight loss or inflammation may require a liquid diet like enteral nutrition, which may offer the small intestine time to heal and improve its ability to absorb nutrients (via The Crohn's & Colitis Foundation). Enteral liquids like Ensure provide calories, macronutrients, vitamins, and minerals, and can be either ingested normally or through a feeding tube. Parenteral diets are used in cases of serious CD complications such as bowel perforations or in preparation for surgery — this method transmits nutrients into the bloodstream through an IV. In less extreme cases, CD sufferers experiencing a flare-up may also benefit from temporarily reducing intake of insoluble fibers that come from plant-based foods such as fruits, vegetables, nuts, seeds, and whole grains (via The Crohn's & Colitis Foundation).
Another diet that may be helpful for some CD patients is the IBD Anti-Inflammatory Diet (IBD-AID), which, according to Nutrition Journal, includes fruits and vegetables, prebiotics from soluble fiber in seeds or oats, fermented foods such as kefir and miso, unsaturated fats from seeds, fish, and olive oil, and lean proteins. This eating plan also pinpoints nutrient deficiencies, food intolerances, and determines the appropriate texture of food a patient can tolerate. The study of the IBD-AID diet on 40 patients saw a reduction in common CD symptoms and medication use in a majority of the participants.
Role of food allergies and sensitivities
An additional question regarding diet and its relationship to CD is the role of food allergies and sensitivities. There doesn't seem to be a correlation between food allergies and CD, other than the possibility that some patients may have both conditions (via The Crohn's & Colitis Foundation).
Some studies do show higher levels of immunoglobulin G (IgG) antibodies in CD patients, indicating a reaction to certain foods, but it's unclear as to whether the potential food sensitivities are affecting the severity of Crohn's, or if the high levels are due to inflammation and a compromised intestinal barrier that come with having Crohn's (World Journal of Clinical Cases). The latter is likely according to a 2019 study in Internal Medicine, which questions the efficacy of IgG antibody testing as a tool for determining food sensitivities, but suggests that the test may offer some insight into the level of intestinal permeability of the patient — also known as leaky gut syndrome, a condition in which not only water and nutrients pass through the walls of the GI tract, but also pathogens like bacteria or viruses, potentially causing GI distress. A 2021 study suggests a relationship between leaky gut syndrome and autoimmune diseases and encourages more research to understand the connection (via Frontiers in Immunology).
The Crohn's & Colitis Foundation suggests only avoiding foods known to contribute to symptoms during flare-ups, being cautious about avoiding too many foods, and replacing triggering foods with others with similar nutrition due to the risk of malnutrition.
Role of vitamin and mineral supplementation
Vitamin and mineral deficiencies in CD patients are common due to decreased nutrient absorption, food avoidances, medication side effects, and surgery (via WebMD). Supplementation may offer prevention of additional concerns such as anemia, osteoporosis, and cognitive decline, which can come with malnutrition. Common micronutrient deficiencies include calcium, vitamin D, magnesium, zinc, iron, and B vitamins like B6, B12, and folate. A quality multi-vitamin is a good starting point, but understanding current levels of these nutrients is helpful in knowing how much to supplement. For example a multi-vitamin may include 1,000 IU or less of vitamin D when as much as 50,000 IU may be necessary for a short period of time, under the guidance of a medical professional, depending on current level and use of certain medications. Vitamin D is necessary for calcium absorption, immunity, cardiovascular health, and muscle and nerve function (via National Institutes of Health Office of Dietary Supplements).
Chewable or liquid supplements may be used if absorbability is impaired or food intake and appetite are low (via The Crohn's & Colitis Foundation). Working with your medical doctor and a nutrition professional is helpful in understanding which supplements you need and how they may be helpful.
Stress management
Living with a chronic health condition can add to the stress of everyday life, and while mental health conditions don't cause IBD, it can contribute to flare-ups and make managing the symptoms even more difficult (via Healthline). Additionally, physical signs of stress like fatigue, nausea, diarrhea, and changes in appetite share similarities to symptoms of Crohn's, making it difficult to assess a patient's progress.
A 2011 study reviewed several stress management techniques such as meditation, deep breathing, and cognitive behavioral therapy, and found that stress management can "lower stress levels ... resulting in reduction of disease symptoms, lowering of biological indicators of disease, prevention of disease and/or improvement of patient's quality of life" (Health Science Journal). A study in Behaviour Research & Therapy sought to determine how stress management affected CD patients, and saw a reduction in some symptoms such as fatigue and abdominal pain. As these studies indicate, including a more holistic approach to Crohn's disease management may offer an improved quality of life.
Therapeutic effects of acupuncture
Acupuncture is a treatment method that has been used for over 2,500 years in ancient Chinese medicine, and involves inserting tiny needles into specific points on the body, stimulating nerves and impacting the immune system as well as circulation — or as Chinese philosophy says, stimulating the qi, the body's life-giving force (via Healthline). It is often used for conditions like chronic back pain and headaches.
A research review in the journal Inflammatory Bowel Diseases reviewed the efficacy of acupuncture to treat those with IBD, looking at specific locations on the body that correlate to IBD symptoms. For example, the acupoint in the webbing between the index finger and thumb correlates to diarrhea and abdominal pain. The researchers saw promising improvements in IBD symptoms, as well as in depression and anxiety, and encouraged more research to fully understand how acupuncture can play a part in CD treatment. Given this, they conclude that acupuncture may offer a safe, complementary therapy to help Crohn's patients manage symptoms and increase overall feelings of wellbeing.