The Skyrizi Commercial Explained: Drug Uses, Side Effects, And More
The Skyrizi commercials spread through 2022 and 2023 talk about the drug's ability to fight inflammation and reduce symptoms of plaque psoriasis, psoriatic arthritis, and Crohn's disease. While the former two carry the tagline "Nothing is Everything" (meaning no skin symptoms and no joint pain is everything), the latter goes with the saying "Control is Everything" (to mean that the medication helps someone control their inflammatory bowel disease or IBD symptoms).
The most common type of psoriasis, plaque psoriasis is an autoimmune condition that causes thick, scaly patches on the skin. It can also manifest in dry and cracked skin that bleeds, itching, burning, and soreness, and rashes that flare every few weeks or months. While the exact cause of the condition is unknown, science points toward a malfunctioning immune system where healthy cells are attacked when they shouldn't be. Psoriatic arthritis is a type of arthritis with a skin rash. It's a chronic autoimmune disease that results in inflamed, swollen, and painful joints. A faulty immune system where healthy cells and tissues are attacked is thought to be the cause here, too. The resulting inflammation is what leads to joint troubles.
Crohn's disease is an inflammatory bowel disease (IBD), which also falls under the umbrella of autoimmune conditions. An abnormal immune system response results in inflammation of the gut and related symptoms like diarrhea, abdominal pain and cramping, mouth sores, fistulas, anal fissures, etc. What is Skyrizi, and how does it help with these conditions?
What is Skyrizi and what does it do?
Skyrizi is the brand name for the prescription medication risankizumab-rzaa (pronounced ris" an kiz' ue mab), which belongs to a type of drug called interleukin-23 (IL-23) antagonists. It works by binding to the naturally occurring cytokine p19 subunit of interleukin-23 (which plays an important role in boosting inflammation) and reducing levels of inflammation in your system.
Manufactured by AbbVie Inc., Skyrizi was approved by the U.S. Food and Drug Administration (FDA) to treat plaque psoriasis in 2019 and for the treatment of psoriatic arthritis and Crohn's disease in 2022. Skyrizi is also a biologic, a type of manmade drug created from living cells that targets inflammation. Skyrizi is not available as a biosimilar (a generic version of a biologic).
According to the drug commercials, a reduction in inflammation means greater relief from symptoms of the autoimmune diseases plaque psoriasis, psoriatic arthritis, and Crohn's disease. More specifically, Skyrizi is meant for the treatment of moderate to severe plaque psoriasis in adults who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy), active psoriatic arthritis in adults, and moderate to severe Crohn's disease in adults. It has not been reviewed for safety or use in children. The biologic starts working immediately after you take your first dose, but it may take a few more doses before you see changes in symptoms.
Skyrizi side effects
In clinical trials, the most common side effects of Skyrizi when used to treat people with Crohn's disease included upper respiratory infections, headache, stomach pain, injection site reactions, fever, back pain, joint pain, low red blood cells (anemia), and urinary tract infection (UTI). In the case of treatment for people with plaque psoriasis and psoriatic arthritis, the common side effects included upper respiratory infections, fatigue, fungal skin infections, headaches, and injection site reactions. In clinical trials, fungal infections were observed in 1.1% and 0.3% of people who took Skyrizi and a placebo, respectively. Infections in general are a result of the medication lowering your immune system response.
Other serious side effects include liver problems in people with Crohn's disease. In fact, according to the Medication Guide and Prescribing Information (which you can visit for an exhaustive list of side effects), when used for treatment on someone with Crohn's disease as an intravenous infusion, Skyrizi was linked with changes in liver blood tests and a rash that led to hospitalization.
You could also develop an allergic reaction to the drug (or its active or inactive ingredients). Apart from the active ingredient risankizumab-rzaa, inactive ingredients include polysorbate 20, sodium succinate, sorbitol, succinic acid, glacial acetic acid, sodium acetate, and trehalose, depending on the dosage and what form the drug comes in. An allergic reaction might manifest as a skin rash, itchiness, flushing, and, more severely (although rare), swelling under your skin near your eyelids, lips, hands, or feet, swelling of the tongue, mouth, or throat, and difficulty breathing. Call your doctor, visit the emergency room, or call 911 if this happens.
Skyrizi dosage: Types, strengths, and more
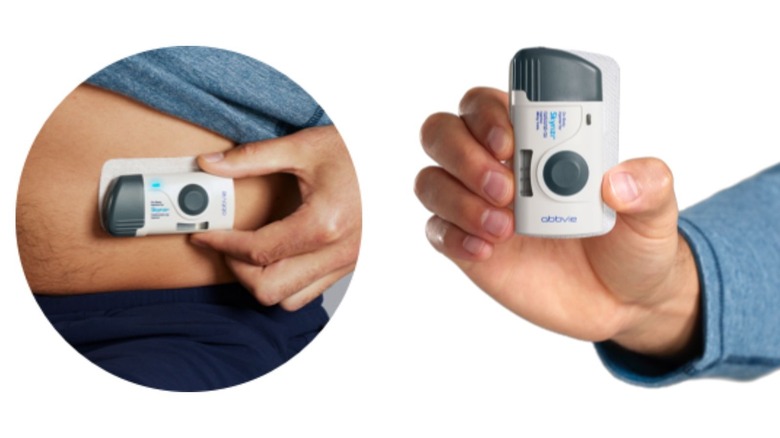
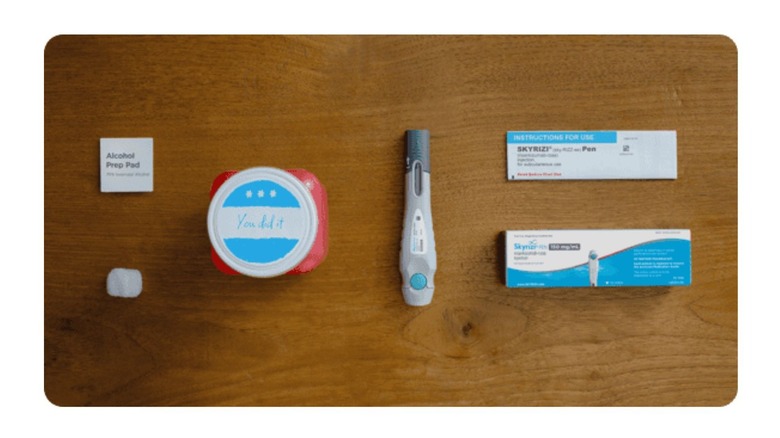
There are broadly two ways in which you can get administered Skyrizi: a subcutaneous injection (which you can administer yourself after the first one is done by your healthcare provider and you're taught how to do it) and an IV infusion (which is administered by a healthcare professional at an infusion clinic or doctor's office). The subcutaneous injection, comes in three forms: a pre-filled injection pen, pre-filled syringe, and on-body injector with a cartridge.
Following are the dosage strengths of each of them:
Injection pen: 150 milligrams per milliliter (mg/mL)
Syringe: 75 mg/0.83 mL and 150 mg/mL
On-body injector with a cartridge: 150 mg/mL (cartridge: 180 mg/1.2 mL and 360 mg/2.4 mL)
IV infusion: 60 mg/mL
Typically, administering Skyrizi starts off with a loading dose (where you're given more of the medication). This is also known as the induction phase. Thereafter, the medication is continued via maintenance doses to keep effective levels of the drug in your system. If you miss a dose, consult with your healthcare provider. For self-administering injections, you might be able to take them as soon as you remember and readjust your schedule per your doctor's instructions. It is recommended, however, that you set up reminders (either on your phone or a calendar) so that you don't miss a dose. Typically considered a long-term treatment, your doctor will be able to shed light on specifics about how Skyrizi fits into your treatment. Don't take more than your prescribed dose, as this can cause serious side effects. Call your doctor or poison control at 800-222-1222 if you do.
Skyrizi dosage: Recommended dosing, storage, and more
The recommended dosage is 150 milligrams via subcutaneous injection at week 0, week 4, and every 12 weeks thereafter for plaque psoriasis; 150 milligrams through subcutaneous injection at week 0, week 4, and every 12 weeks thereafter for psoriatic arthritis; and an induction dosage of 600 milligrams via intravenous infusion over a period of one hour at week 0, week 4, and week 8 and maintenance dosages of 180 milligrams or 360 milligrams through subcutaneous injection at week 12 and every 8 weeks thereafter for Crohn's disease. However, your healthcare provider will be the best one to judge what course of treatment and what dosages would be suited for you.
For administration of the subcutaneous injection, there are steps to follow, like proper handling of the injection pen, how to clean the site of injection, how to inject, and how to discard the used syringe/pen. Your doctor will guide you on the proper steps to follow.
The drug starts working immediately, but it can take up to four weeks (in patients with Crohn's disease) to see improvement in symptoms. Clinical trials showed less abdominal pain and fewer bowel movements at one year and positive changes in the intestinal lining in 12 weeks. For plaque psoriasis and psoriatic arthritis, you could start to see changes in symptoms in four weeks. For more details on the clinical trials and symptom relief time, read the Prescribing Information. Store Skyrizi in the refrigerator between 36° and 46°Fahrenheit. Do not freeze or shake the drug and keep it in the original package to protect it from light.
Skyrizi precautions
Skyrizi weakens your immune system, which is one of the main concerns when it comes to the biologic. Your likelihood of developing infections increases with this medication. Typically, your doctor will check you for infections and tuberculosis (TB) before starting treatment with Skyrizi and treat you for TB (if you have it) before starting you on the drug. They will also watch for signs of TB during and after treatment. Alert your doctor if you experience any signs of an infection while on Skyrizi, like fever, sweats, or chills, coughing, shortness of breath, bloody mucus (phlegm), muscle pain, weight loss, diarrhea, stomach cramps, painful urination, frequent urination, or warm, red, or painful sores on your body (unlike your psoriasis).
It is important that you discuss any underlying health conditions, particularly infections, with your healthcare provider before you start taking Skyrizi. Even if you've come into close contact with someone with TB, your doctor should know about this. Other details to disclose to your doctor include when you recently received (or are going to receive) a vaccine, whether you're pregnant or planning to become pregnant, and if you're breastfeeding or plan to breastfeed. Immunization can mess with your immunity some more and put you at risk for infection. Your doctor should also know about any other prescription medications, over-the-counter drugs, vitamins, or herbal supplements you're taking.
This is not an exhaustive list of precautions. For more information, read the Warnings and Precautions section under Prescribing Information.
Avoid taking Skyrizi if you are on these medications
While there is no data on any prescription medications Skyrizi could interact negatively with, this doesn't mean you shouldn't talk about any medications you're on with your healthcare provider.
The biologic can, however, interact with certain live vaccines (as noted above). Vaccines to be mindful of include:
- typhoid
- smallpox
- chickenpox
- yellow fever
- measles, mumps, and rubella (MMR)
- intranasal flu (FluMist)
- rotavirus
Drinking alcohol was not considered a concern with the biologic, but alcohol consumption can worsen symptoms of psoriasis, so this is something to keep in mind. Additionally, alcohol also lowers your immune system response, so you may want to tread with caution when it comes to taking a biologic to weaken your immune system and alcohol at the same time. Run these concerns by your doctor just to make sure you're doing the right thing for your body while on a Skyrizi treatment plan.
How much does Skyrizi cost?
What you will pay for Skyrizi will depend on your access to the drug, the insurance you have, and how much coverage you get from it. The Wholesale Acquisition Price (WAC), also known as the list price (which is not reflective of what you will pay as a patient), is $21,017.36 for one dose of Skyrizi.
That being said, discussing what it will cost by speaking with your insurance provider, pharmacy, and doctor is the best way to go about things. Note that you might require prior authorization from your insurance provider to see if the drug is covered. This is a job for your doctor and insurance provider. After they discuss particulars, authorization can be approved.
You can also check out the details on the Skyrizi Complete Savings Card provided by the drug company and see if you're eligible. This offer is only available for eligible commercially insured patients. The drugmaker's assistance program, myAbbVie Assist, is another resource to look up. For a better understanding of the insurance you have and how much financial assistance you can get, you can visit the NeedyMeds and Medicine Assistance Tool websites.
Skyrizi vs Humira: What's the difference?
Humira (generic name: adalimumab) is another biologic used in the treatment of Crohn's, psoriatic arthritis, and plaque psoriasis. Unlike Skyrizi, however, Humira is approved for use to treat other conditions as well, like ankylosing spondylitis, moderate to severe hidradenitis suppurativa, moderate to severe rheumatoid arthritis, moderate to severe ulcerative colitis, and sometimes uveitis. Skyrizi is currently being studied for the treatment of ulcerative colitis. Humira can also be prescribed for some children's use.
Unlike Skyrizi, Humira is available in biosimilar form. Humira works a little differently than Skyrizi, in that it's known as an TNF-alfa (alpha) inhibitor; it binds to the signalling protein TNF-alfa, which is released during inflammation, and blocks its action, thereby reducing inflammation and tissue damage.
Boxed warnings for Humira include a weak immune system (like with Skyrizi). Your risk of developing an infection, particularly pneumonia and TB, goes up with the use of this biologic. Just like with Skyrizi treatment, your healthcare provider will screen you for infections before starting you on the medication. Humira also comes with the added risk of developing blood cancer, colon cancer, and breast cancer. Side effects of using Humira include serious infections, hepatitis B infection in carriers of the virus, allergic reactions to the drug and its ingredients, nervous system problems like numbness and tingling, vision problems, weakness in arms and legs, dizziness, bruising or bleeding, heart failure, and liver problems. But these are not all the side effects associated with Humira. A complete list can be found under Medication Guide.