The Tremfya Commercial Explained: Drug Uses, Side Effects, And More
The Tremfya (trem-FYE-ah) ad starts with a woman in a dark room with patches of her skin seemingly on fire. Another woman touches her arm, feeling her joints locked in a metal brace. Tremfya (guselkumab) comes to the rescue to help them break free of their plaque psoriasis and psoriatic arthritis to "Emerge as you."
Plaque psoriasis is an autoimmune disease that affects more than 6.5 million people (per Cleveland Clinic). The plaques that develop on the skin can itch, bleed, or be discolored. People who have psoriasis could develop psoriatic arthritis, where they will feel inflammation and pain in their joints. Tremfya treats moderate-to-severe plaque psoriasis and active psoriatic arthritis.
The drug, manufactured by Janssen Biotech, works by blocking interleukin-23 — a protein that signals inflammation that triggers psoriasis symptoms such as itching and pain. The clinical studies showed that 70% of people with moderate-to-severe plaque psoriasis saw 90% clearer skin after 16 weeks. More people noticed clearer skin after 28 weeks. More than half of people with active psoriatic arthritis noticed a 20% relief of their joint pain, stiffness, and swelling after 24 weeks. Relief from psoriatic arthritis endured for two years after taking Tremfya.
Tremfya side effects
Tremfya has several common side effects. In the clinical studies, some people experienced upper respiratory infections, headaches, or pain at the injection site. Fewer people developed joint pain, stomach flu, and diarrhea. Others developed herpes simplex infections and fungal infections on the skin.
Some people experienced more serious side effects such as a hypersensitivity of the immune system that required hospitalization. You should stop using Tremfya if you develop symptoms such as trouble breathing, itching, tightness in your chest, lightheadedness, or swelling in your throat, mouth, or face. Because Tremfya could weaken your immune system, the drug also increases your risk of infection or tuberculosis. Call your doctor if you develop fever, muscle aches, burning during urination, diarrhea, or other symptoms of an infection. For a list of all the possible side effects, visit the Tremfya website. You should also make your doctor aware of any side effects you might experience while using Tremfya.
Tremfya dosage
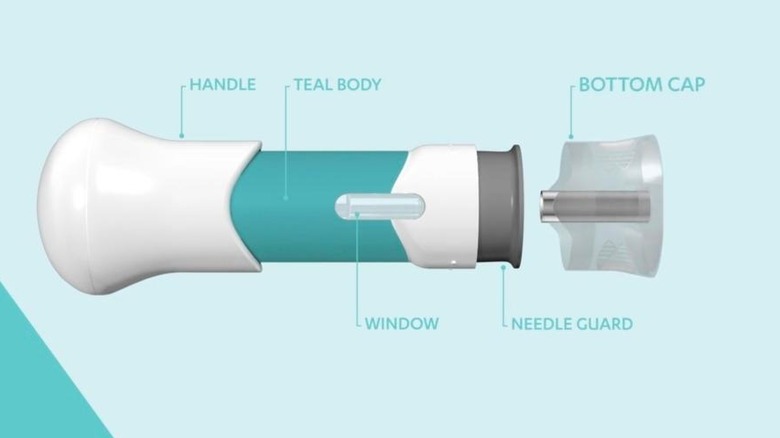
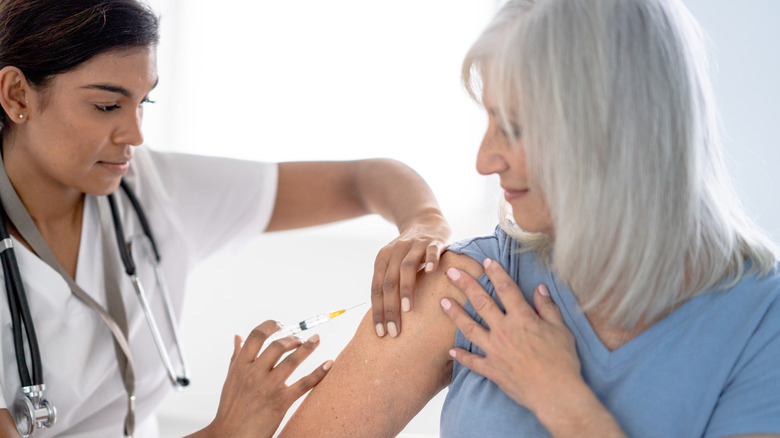
Both plaque psoriasis and psoriatic arthritis require a 100-milligram injection into your skin at the start, then another injection four weeks later. After these two doses, you'll continue administering your injections once every eight weeks. Tremfya only comes in one strength that's prefilled with 100 milligrams of the medicine, and the syringe can only be used once. If someone accidentally injects Tremfya or you inject more than prescribed, call Poison Control at 1-800-222-1222. You can also get help online at the Poison Control's website.
While a medical professional can administer your injection for you, you can give the injection yourself. Before doing this, you should be trained on the proper way to administer the shot and properly dispose of the syringe in a container approved by the Food and Drug Administration. Even if you're trained to administer your injection, details are available at the Tremfya website's Instructions for Use.
Tremfya precautions
Because Tremfya increases your risk of infections, you should let your doctor know if you have a medical history of infection or have a recurring infection. You should avoid Tremfya if you have tuberculosis or have recently been in contact with a person with tuberculosis, such as while traveling. Your doctor will probably test you for tuberculosis before you get treated with Tremfya.
You should also be up-to-date with any live vaccines such as the MMR vaccine, rotavirus, smallpox, or chickenpox. You can't be receiving any of these live vaccines while being treated with Tremfya. Vaccines to protect against the flu (other than the nasal FluMist), COVID-19, shingles, or tetanus are not live vaccines and can be administered while you're using Tremfya.
Although it's not known if Tremfya can harm your baby or be passed through breast milk, it's best to let your doctor know if you're nursing, pregnant, or plan to become pregnant.
Avoid taking Tremfya if you are on these medications
Certain types of inflammation-related proteins can affect the production of CYP450 enzymes that metabolize certain medications. Even though the research shows that Tremfya doesn't affect these enzymes, it's not clear if taking Tremfya might impact the effectiveness or side effects associated with drugs that are metabolized by the CYP2D6 enzyme. Although the possibility for interaction is low, the drugs that could have a potential for interaction include:
- Antidepressants
- Antipsychotics
- Some types of antiarrhythmics
- Some beta-blockers
- Opioids
Drugs.com says 41 drugs are considered to have a major interaction with Tremfya. Humira, another immunosuppressive drug to treat psoriatic arthritis, is the only drug listed as a major interaction among the frequently checked drugs. Amlodipine and atorvastatin are also listed as having potentially moderate interactions with Tremfya because Tremfya could reduce the amount of these medications in your bloodstream. You might need to adjust the dosage of these medications while being treated with Tremfya. To be safe, it's best to consult with your doctor about all your medications before taking Tremfya and check for any potential interactions.
How much does Tremfya cost?
Because Tremfya is a biologic drug made from living cells and considered a specialty drug only available in certain pharmacies, it's considerably more expensive than other drugs that treat psoriasis. In 2023, the list price for Tremfya is $13,212.19 for a single 100-milligram injection before pharmacy fees and administration costs. There isn't a generic version for Tremfya, so GoodRx's lowest price is $13,326.
Janssen also has a Tremfya with Me program to help those with commercial or private insurance cover their out-of-pocket cost for their medication. Depending on your eligibility, you could pay just $5 for each Tremfya injection. This program is not available for those on Medicare, Medicaid, or other government-funded programs. However, those who are on the Health Insurance Marketplace (aka "Obamacare") could be eligible. For those who don't have insurance or have government-funded insurance, Janssen has a Janssen CarePath program to help pay for Tremfya.
Tremfya vs. Humira
Humira (hyoo-MEE-ruh) is another FDA-approved medication to treat both plaque psoriasis and psoriatic arthritis. Humira, whose generic name is adalimumab, also treats rheumatoid arthritis, Crohn's disease, and ulcerative colitis. Both drugs are injections, but you'll take Humira once every two weeks after the two initial starter doses. Like Tremfya, you'll still be at risk for infections, headaches, and joint pain with Humira, but you could also see side effects such as high cholesterol, high blood pressure, or urinary tract infections. More serious side effects that could occur with Humira could include heart failure, multiple sclerosis, or some types of cancer. Humira has a boxed warning for its risk of cancer and serious infection.
You won't pay as much for Humira per dose as you would with Tremfya. You can find a two-dose kit of Humira for about $7,300 on Drugs.com, which comes to $3,650 per dose. Remember that you take Humira more often than Tremfya, so you might wind up paying more over time. Although Tremfya doesn't have a generic available, nine drugs were approved by the FDA that are biosimilar to Humira (per GoodRx). Cyltezo (sil-TEE-zoh), whose generic name is adalimumab-adbm, and Abrilada (AH-brill-ah-dah), whose generic name is adalimumab-afz, are considered to be interchangeable with Humira. However, Abrilada sells for considerably less than Humira. You'll have to check with your doctor to make sure these biosimilars treat your condition, and your insurance company might not cover biosimilars.